This activity compliments the teacher’s lesson on half-lives of radioisotopes.
Materials:
Quarters, calculator, half-life table (provided)
Helpful visual aid: Decay charts of different radioisotopes
Procedure:
The teacher will hand out quarters to each student. If there are an odd number of students, the teacher will also participate.
Question: Statistically, what are the chances of a flipped quarter landing on heads? Tails?
***Statistically, a quarter has a 50% of landing on heads or tails
Explanation: HALF-LIFE is the statistical time it takes for a RADIOISOTOPE (any isotope that is radioactive) to reduce its starting quantity by half. For example, if you have 100g of plutonium-239, it will take about 24,000 years for the plutonium to reduce to 50g. The half-life of a radioactive element is an immutable characteristic. That is, the half-life does not change, regardless of temperature, climate, pressure, environment, etc…
Explanation: For this activity, each quarter represents an atom’s nucleus. If the quarter lands on HEADS, then that “nucleus” did not decay. If the quarter lands on TAILS, then the “nucleus” did decay. Each round the quarters are flipped represents an unspecified unit of time.
***Students or teacher can choose the unit of time each round represents (seconds, minutes, hours, months, years, etc)
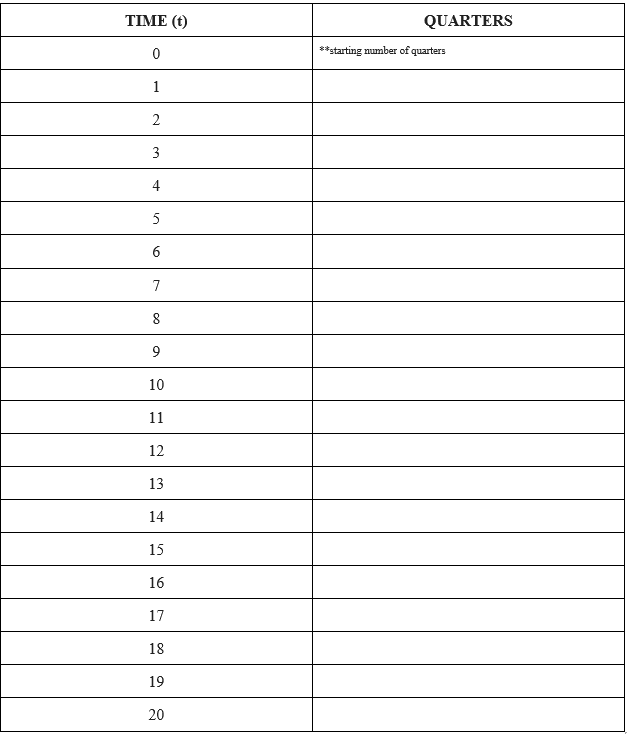
Activity: On the teacher’s command, students will flip their quarters. HEADS, the quarter will be flipped again in the next round (the “nucleus” did not decay). TAILS, the quarter will be taken out of circulation (the “nucleus” decayed). The teacher will repeat the procedure until all quarters have landed on TAILS. During each round, the teacher or a student volunteer will record the number of quarters that landed on HEADS (the “nucleus” that did not decay) in the chart provided. Each round the students flip the coins will equal time. t0 will be the starting number of quarters. For example, if there are 20 quarters starting, then t0 will equal 20 quarters.
Question: If everyone in the class flips their quarters, statistically, how many should land on heads? Tails?
***If there are 20 quarters being flipped, then 10 should land on heads, and 10 should land on tails.
Explanation: Unless by a statistical miracle, the quarters did not land 50% heads and 50% tails in each round. That is because statistics and probability inform us of what is likely to happen but not what will actually happen. If we were to continue flipping quarters forever, we will get closer and closer to that 50% heads and 50% tails probability. Half-lives work the same exact way. We expect that 100g of plutonium-239 will become 50g in in 24,000 years. However, radioactive decay is spontaneous. We don’t know which atoms will decay and when. But in 24,000 years, we do expect that 100g of plutonium-239 will reduce to approximately 50g.
Explanation: Each radioisotope has its own half-life. For example, plutonium-239 has a half-life of about 24,000 years, but plutonium-240 has a half-life of about 6,500 years. Uranium-234 has a half-life of about 250,000 years while uranium-235 has a half-life of about 710,000,000 years. Iodine-131, on the other hand, has a half-life of about 8 days. Here is a list of radioactive isotopes, their half-lives, and their decay types.
***Helpful visual aid: Decay charts of different radioactive isotopes
Activity: The teacher will call on a student to plot the results on the board. X-axis is TIME and the Y-axis is QUARTERS.
Question: What type of graph is this?
***It is a logarithmic graph. (Good segue into calculating half-lives and the half-life formula)
RESOURCES CONSULTED
Cahill, Christopher, and James “August” Ridenour. “Laboratory #2–Radioactive Decay Laboratory.” Lab Exercise, Elliott School–The George Washington University, 2018.
Cahill, Christopher, and James “August” Ridenour. “Lecture#3.” Lecture presented at the Elliott School–The George Washington University, Washington, D.C., September 2018.
Cahill, Christopher, and James “August” Ridenour. “Lecture#4.” Lecture presented at the Elliott School–The George Washington University, Washington, D.C., September 2018.