Stephane Groueff: Recording from Wilmington, Delaware. DuPont Company.
Samuel McNeight: I’ll say the major part of the reason why I ask Dale to come over with me was that Dale’s acquaintanceship and part in the Manhattan Project considerably pre-dates mine. Also, he was a part of the reactor group, which I was not. I had nothing to do with reactors.
Groueff: You had to do with the separations?
McNeight: The separations plants entirely.
Groueff: In Hanford?
McNeight: This is right. They were well on their way to construction before I arrived there.
Groueff: But were there any pilot plants before, or how did you learn about separation before you built the plant in Hanford?
McNeight: Well actually, the plant at Clinton Laboratories that was at Oak Ridge was more or less a pilot plant.
Groueff: I see. You first built the Oak Ridge plant?
McNeight: This is right.
Groueff: The reactor plus the separation plant?
McNeight: Well, there was a reactor built at Oak Ridge, but I doubt if you could call it a pilot reactor.
Dale Babcock: We called it a pilot reactor.
Groueff: It never served as a pilot because the other one was built simultaneously, no?
Babcock: No. The method of cooling was entirely different. The Oak Ridge one was cooled with air, whereas the Hanford one was cooled with liquid water.
Groueff: Water.
Babcock: And then the construction was tremendously different in each case.
Groueff: But was it at the beginning in Chicago they had discussed the cooling problem was one of the most important, no? They discussed the helium?
Babcock: Yes. When the water company was originally brought into the operation by the Chicago group, helium was the preferred coolant. When we first started on the operation, our engineering department was working using helium as the coolant. Then Wigner’s group: Gale Young and [Alvin] Weinberg and John Wheeler—I can’t remember the rest of the people—had been working on the concept of cooling with light water. Now you see, the problem was not only an engineering one, but also a physics one, because light water introduces into the reactor a poison which makes the reactor shut down.
Groueff: Is that xenon?
Babcock: No.
Groueff: No? I’ll ask you later about that problem later.
Babcock: The reactivity, or whether there was enough neutrons being generated to continue the reaction or whether the thing would die out, was an extremely delicate balance. We knew that we just barely had enough to make the thing go. Well now then if you add the water that’s required for the coolant, you may have over tipped the balance and it won’t work. Well as it so happened, it was very close, but the water was alright. Now then, this was the thing that the early people at Chicago worked out.
Groueff: The Wigner group?
Babcock: The Wigner group. And there was a very famous report that they put out, I’ve forgotten what the title of it was, but it described the operation of a reactor—which is cooled with light water. And we then looked at that, and I guess Crawford Greenewalt was the one who had the major decision to say we accept light water cooling. And from that day on, the DuPont Company then did all of its work on light water cooling, abandoning the helium.
Groueff: The helium.
Babcock: Now the reason I’ve gone into that is if we had used helium cooling, the air cooling that was used down at Oak Ridge would have been a perfectly reasonable pilot plant for that. But when we went to the water cooling, the problems were so different that the plant at Oak Ridge—
Groueff: The Hanford reactor was a completely new thing?
Babcock: A completely new venture as far as that portion of it is concerned, yes.
Groueff: What was the cooling of the Fermi reactor—the first one in Chicago?
Babcock: None.
Groueff: None. I see.
McNeight: It didn’t run at high enough levels to regenerate much heat.
Babcock: The amount of heat that it was – I believe that it may have eventually, at one time for a short length of time, put out as much as one kilowatt. Normally it was about as much as a few watts.
Groueff: Alright, so you didn’t need cooling?
Babcock: Your body right now is putting out 300 watts.
Groueff: Why was the air-cooling chosen for Hanford?
McNeight: Why was it not chosen?
Groueff: Yes. No you said that in Hanford, you used the air?
Babcock: No. At Oak Ridge we used air.
Groueff: Oak Ridge. Oak Ridge was air and Hanford was water.
Babcock: Hanford was water.
Groueff: That’s it. That was the Columbia River water. And that was quite a problem, I understand, because—
Babcock: There were a number of problems that came up on account of using water, yes.
McNeight: Actually, that was one of the reasons, I believe, for the site selection—the availability of a lot of water.
Groueff: Of water.
McNeight: Good, cool water. Are you ready for a little anecdote now?
Groueff: Yes.
Babcock: One of the reasons that we selected Hanford was because of the very large supply of not only cold water, but relatively clean water as well. If you’ve seen the Columbia water, it’s largely sparkling blue—at least compared to the Mississippi or the Savannah River. We thought that starting out with this nice, clean water that it would not be a very great problem to clean the water up a little bit further, and we could then put the water through the reactors. That is the basic assumption that we started on. Well, I believe that the person that beat the drum the most for doing test work on the water was Charles Cooper and George Graves. They said—
Groueff: From DuPont?
Babcock: Yes. Their interest was to get a test operation going using the Columbia River water and the type of purification that we had planned to use, and simulated heating elements—which would simulate the heat of the reactor—and just see what happens. Well quite to everyone’s surprise, a gelatinous film formed on the heating elements—or which would be the fuel elements of a reactor. The film grew so rapidly that the flow of water was shut down to nearly zero after about a day or so.
Well now obviously you can’t have that kind of a thing happening, which meant that our water treatment was inadequate. A water treatment system had to be developed more or less immediately to keep this film from forming. It was developed, and the film was eliminated.
Groueff: But where did this film come from? From impurities or—
Babcock: The water that was prepared from the Columbia River was nice, clean drinking water. You could hold it up to the light and it was sparkling clear, but there were enough dissolved chemicals in them that when they were heated up in the presence of an aluminum jacket, they would make a chemical reaction that generated this gelatinous material.
Groueff: So it wasn’t as pure as you thought?
Babcock: Well as you know, the purity of water is a kind of an evanescent thing. We talk about pure water being pure enough to drink. But as far as a chemical is concerned, it isn’t very pure—there’s a lot of dissolved salts and whatnot in it. It was these dissolved materials, which normally you wouldn’t be concerned about, that gave us the problem. Now then to carry this story further, when we went down to Savannah River – have you seen the Savannah River?
Groueff: No.
Babcock: It is blacker than coffee. We thought we were going to have a very severe problem with water—this type of water, since it was so muddy looking to start with. But quite to our surprise, the best treatment for that water was just to leave it alone. So they put the mud and everything right through the cooler.
Groueff: So purity is a very relevant thing?
Babcock: Purity is relative thing, and not something that can be judged by the eye.
Groueff: But was there any contamination danger for the population there? And also, the salmon fish, which is quite a problem there, how did you solve this problem with the water?
Babcock: Well there are two ways that the problem was solved. If you put water through a reactor, and this water contains almost any of the dissolved elements, these elements will become radioactive. The more dissolved materials there are there, the more radioactive the water will become. This film that I was speaking about became radioactive, and other materials in there also became radioactive. We took the water from the reactor and ran it into a retention basin. The storage time in the retention basin, as I remember it, was about eight hours.
In fact, we had two retention basins so that if we got into trouble with an unusually large amount of contamination, we could switch from one basin to the other. Well, the eight hours retaining time allowed the major portion of the radioactive materials to decay away. So the amount of material that was left was greatly reduced. However, it is true that there were still radioactive materials there and they were picked up by the various plant organisms, which in turn were eaten by the fish. The fish became very mildly contaminated with radioactivity.
McNeight: Actually, to pursue that a little bit further, one of the early things that was built at Hanford was, let’s say, what’s called a fish laboratory.
Babcock: Yes.
Groueff: To study how to protect the fish?
Babcock: To study the reactions that the fish had to this water, and the fish under—
Groueff: You had a special laboratory for the fish?
Babcock: This is true.
Groueff: Was this DuPont?
Babcock: It was started during DuPont operation. I believe it’s still in operation. I believe they still have a fish laboratory—it’s part of the biology.
Groueff: When you build atomic bomb, to think of the fish also – it’s quite a good detail.
McNeight: To pursue the water subject a bit further, isn’t it true, Dale, that the first thing that was put in operation at Hanford was the water study laboratory down on the banks of the river?
Babcock: Yes. Somewhat amusing sidelight is an atomic reactor, as you know, puts out tremendous quantities of heat.
Groueff: Yes.
Babcock: Although we were only testing just a very small portion of an atomic reactor—two or three fuel channels to be simulated—it took a large amount of heat to do this simulation. The way we got this heat was to bring in five steam locomotives, which were incapable of pulling a train but were capable of generating steam. I don’t know whether they came in on their own wheels or not.
At any rate, they were set up on the banks of the Columbia there without their wheels. They were fired though by locomotive firemen, and we generated enough steam to heat these simulated reactor tubes to the same temperature on the surface of which they would have been heated in a reactor with uranium.
Groueff: That’s very interesting. I can imagine the sight of five locomotives there. Of course, the area was classified and the population wasn’t watching the trains?
Babcock: Population was gone.
Groueff: Strange things going on. Tell me, what was your particular job and when did you go first there?
Babcock: Well I was one of the first people on the operation. I will give you the organization. Roger Williams was the assistant general manager of the explosive department, but his particular assignment was I’m going to use atomic energy division. We called it, in those days, the TNX division for secrecy. The organization under Roger Williams split two ways. R.M. Evans, who was called Monty Evans, was the head of the manufacturing organization. Crawford Greenewalt was the head of the technical division.
I was in the technical division. Greenewalt’s assistant director was George Graves and then there were four chiefs or flunkies or whatever you want to call them that operated under Greenewalt and Graves. They were Hood Worthington, who was in charge of engineering; Lombard Squires, who was in charge of the separations end of the business—what was the guy’s name from Eastern Lab that went down to Oak Ridge?
McNeight: I’m not sure that I even ever knew, Dale.
Babcock: Let me go back—he had the director of Eastern Laboratory.
McNeight: Bill Kirst.
Babcock: Bill Kirst. Bill Kirst. K-I-R-S-T.
Groueff: Kirst?
Babcock: Yeah. He was the technical division’s representative at Oak Ridge. I had charge of physics.
Groueff: Are you a physicist?
Babcock: Physical chemist.
Groueff: Physical chemist. And you were one of the first people to arrive there?
McNeight: Well you are talking prior to any operations people being at Hanford now, aren’t you Dale?
Babcock: Oh yes. I was taken into the organization when it was formed. I believe it was December—
Groueff: ‘42.
Babcock: December ‘42, yes.
Groueff: Yeah. Here, right?
Babcock: Here in Wilmington. Yes.
Groueff: You were employee of DuPont Wilmington and they transferred you later to Hanford?
Babcock: No. I was always in Wilmington.
Groueff: Always in Wilmington?
Babcock: Although I was out at Hanford on trips, and I think I was out there six months at one stay. But I was always—
Groueff: Stationed here?
Babcock: My headquarters were always here.
Groueff: I see. You didn’t live there.
Babcock: No, unless you call being in the transit quarters living. I believe that you stated it correctly.
Groueff: But as far as reactors go, what were the biggest difficulties there at Hanford?
Babcock: Well quite obviously, when you’re building a new operation such as a reactor, and you have very little background to go on, the thing that we were concerned about was, you might say, of different categories. One was the engineering problems—just the plain problem of spending, well, several hundred million dollars in the short length of time that we did. Not having a true pilot plant to go on poses a tremendous problem. That was problem number one.
Problem number two was the physics of the reactor was largely unknown. I must be fair to the people that had done the work up to the time we came in, brilliant work, excellent. But still, the questions that we wanted to know, we didn’t have the complete answers to. The one thing that we wanted more than anything else was what would be the multiplication of the neutrons in the reactor? In other words, would the reactor always be able to run, or would there be conditions when we wouldn’t have enough neutrons available to run it? This was an item that not only we worried about, of course the people at Chicago—Fermi and Wheeler.
Groueff: Yeah.
Babcock: We worried a great deal about that. But they had done the very best they could. They had done the best experiments that they knew how, but the equipment and the number of neutrons that were available to them at that time were inadequate to do a complete study. Therefore, we were going along pretty much on faith and hope that everything would turn out right.
One of the things that we and DuPont insisted on was to get as much reactivity as we possibly could within the framework of a relatively inexpensive and easy-to-build reactor. So whenever changes were contemplated, we always asked ourselves, “Can we get some more reactivity, or should we use some of this reactivity we already have in order to gain an item that is desired?” [This was] largely in the realm of safety, but it was always a balance between would we have enough reactivity or should we use some of the reactivity that we have parasitically to do things that we wanted to do. I would say that that was a large physics question that concerned us.
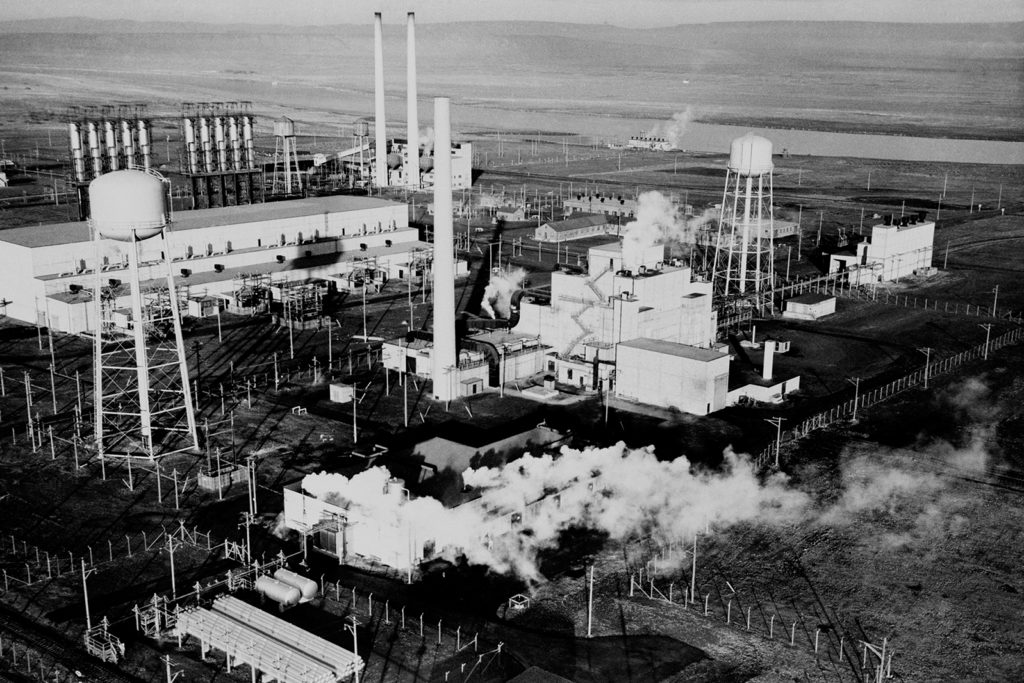
Groueff: Now was it a graphite reactor? The moderator was—
Babcock: The reactor was graphite.
Groueff: Graphite. Yes.
Babcock: Made up of—I don’t know what the right word to use is—the cross-section of the pieces of graphite was square. Did I give the size of it? I think I can, can’t I?
McNeight: I’m not sure whether this has been cleared yet or not on Hanford—the original specs.
Babcock: [It was] about so big on the side and about so long. These were stacked up in a symmetrical pattern cross laid so that—
Groueff: Yeah, like a pile.
Babcock: A freestanding pile. And that’s where the name “pile,” of course, came from. Every other piece of graphite was pierced by a hole, approximately two inches in diameter. Through this hole, we placed an aluminum tube. On the inside of that aluminum tube, we had a fuel element. The space between the aluminum tube and the fuel element was surrounded by cooling water.
Groueff: The cooling water. Yeah.
Babcock: The cooling water flowed down this annulus at a relatively high velocity and cooling—
Groueff: That’s what you called a slug, slugs.
Babcock: Slugs, yes.
Groueff: Now was the aluminum put there in order to separate the uranium from the water to avoid corrosion?
Babcock: In the first place, we put the aluminum tubes in the reactor to confine the water to this channel. If we didn’t do that, the water would get out and flood the graphite and use up more reactivity than we would like.
Groueff: But the space for the water was very little? I mean, not even an inch or something, it was—
Babcock: Oh, not even an eighth of an inch.
Groueff: I see. So that was one of the difficulties of plugging or—
Babcock: We had to have the thickness of the water channel very small in order to reduce the amount of water in the reactor in order to not have too much parasitic absorption of neutrons. Light water is a rather forceful absorber of neutrons. The reactivity of the reactor that I was speaking about which is the number of neutrons that can remain alive was a very strong function of the amount of water that was in there. So we cut this down to what we thought was a near minimum.
Groueff: I saw in the books that I read that one of the main difficulties was the canning of the slugs. I don’t know anything about the technical aspect of that, but why is it so difficult to put this aluminum coating around the uranium? Was it an incredible difficulty?
Babcock: There are two things that we wanted to accomplish by this. One of them, we wanted to have the aluminum can or the aluminum jacket fit so tightly around the uranium slug so that the flow of heat would be very easy from the uranium out through the aluminum jacket to the water. If the uranium slug fit in there loosely so that there was an air gap in there, the uranium slug would get relatively hot, and the air jacket would act as an insulating space.
So therefore we wanted what we call a metallurgical bond or a solid metal path between the uranium and the aluminum. Now then, the route that was chosen was to use—I’m going to use the word solder—aluminum solder to fill the space between the aluminum can and the uranium slug. This space was several thousandths of an inch—variable depending on the exact size of the cans. But the problem of wanting to get this intimate contact, or this metallic contact, between the slug and the uranium was one part.
The other part was to have the aluminum jacket so perfect that there would be no pinholes in it at any place, because if there were a pinhole, water would seep in. Water reacts with uranium quite rapidly. The uranium, when it reacts with water, makes uranium oxide. That swells up. So we then would have the can split open rather rapidly.
Groueff: So it had to be perfect. Absolutely perfect.
Babcock: It had to be an extremely high degree of perfection.
Groueff: Who did this job—DuPont, or you gave to some subcontractors?
Babcock: It was almost entirely done by DuPont. Of course, the Metallurgical Laboratory at the University of Chicago had a large portion in this work.
Groueff: Yeah, but the manufacturing of slugs was done where?
Babcock: At Hanford.
Groueff: At Hanford by a special DuPont people?
Babcock: By DuPont people.
Groueff: That was one of the main difficulties, to find this nearly perfect canning method?
Babcock: The canning method, yes.
Groueff: Did that exist in the metallurgy or in technology as was developed in 1942-43? Was there any precedent of such?
Babcock: Well, you must remember that the amount of uranium metal that was in existence before 1943 was probably about as much metal as on the top of your desk there now. A pound or two—something like that—and we were talking about uranium metal in ton quantities. In fact, hundreds of tons quantities. So what was known about uranium metal was very little. In fact, they didn’t even know what the density was. The density was wrong; it was denser than had been expected by several percent.
The problem with canning was again twofold. The very early solution to the problem was to get the type of solder that was used, which was aluminum silicon—approximately ninety parts of aluminum and ten parts of silicon. This was the material that was found very early and was kept right on through. In fact, I guess they still use it.
Groueff: All this was found between Chicago’s laboratories and DuPont’s people?
Babcock: I think Grasselli did it, didn’t they?
McNeight: I think, yes. At least some of this work was done by the DuPont-Grasselli laboratories in Cleveland.
Babcock: That is right.
McNeight: I don’t know exactly where it is.
Groueff: But the manufacturing, when you started producing it, was done there, not at Wilmington?
McNeight: At Hanford on site.
Babcock: Now then, the problem after you have decided on the geometry of your fuel slug—that’s a uranium cylinder about one inch in diameter and about eight inches long, surrounded by an aluminum can, reasonably tight fitting, but the space between filled with aluminum silicon solder.
Then the problem was to assemble this with the perfection that I spoke of. You have two things that you have to do. One of them is the uranium metal has to be bonded to the can so that there aren’t gaps or splits or bulbs in it.
The second thing you have to have is the aluminum jacket so tightly welded up so that there are no holes or cracks or places for water to get in. Now then, the first problem was to get a perfection of bonding. The way we attempted to solve that was to take a can of aluminum that we poured in about an inch of solder into the bottom of it and then forced the slug into it. The solder then filled the space between.
Well with this operation, sometimes all the air would be taken out, sometimes it would not be taken out. There would be air trapped in there, and we got poor canning. We called this poor bonding or poor bond strength—it could be that the bond was inadequate to start with, or it could be that there was air in there and there was no bond at all. Dr. Raymond Grills, who is now in our film department, made the invention that allowed canning to go along.
Groueff: Grills.
Babcock: Grills. G-R-I-L-L-S. He said, “Let’s do submarine canning.” You take the aluminum can and you put it down in a vat of molten solder. The can then fills up completely with solder. Then you take the uranium slug, which has already been tinned on the outside, and you force it into the can when it is underneath the entire molten material.
Groueff: Without any air?
Babcock: Yes, without any air. And that, what seems like a perfectly simple and easy solution to come to at the time Ray made that suggestion, was not an easy one to come by. From that day on, the canning problem was on the downhill phase.
Groueff: How did you handle the uranium slugs? By some tools?
Babcock: Yes they were—
Groueff: You couldn’t touch them.
Babcock: Let’s see, what do you call them? Tongs I guess.
Groueff: Tongs.
Babcock: Tongs.
Groueff: All the work on the slugs were done by tongs, so behind the screen or behind the—
McNeight: No, no. It’s not necessary to be behind a screen when handling enriched uranium.
Groueff: Uranium is not that radioactive?
Babcock: No. You had to have the surface of the uranium slug prepared very well so that, while it’s a tinning operation although actually it wasn’t—
McNeight: I guess we actually did use tin at some point. At one time that was tried too.
Babcock: What we did was we had I believe it was three baths: a tin, a lead, and aluminum silicate. Isn’t that right?
McNeight: That is right. Don’t go into too much detail on this. This is still not cleared, Dale. The reason it isn’t is it’s still a tricky operation.
Babcock: At any rate, what we did was we prepared the surface so that when the uranium slug was slipped into the aluminum can, a metallurgical bond was between the uranium and the molten solder, and then between the molten solder and the solid can. This metal contact, then, was maintained throughout.
Groueff: I see. During the cooling operation, did the temperature of the whole river increase? I mean with all the water going?
Babcock: Well in the first place, only a small fraction of the Columbia River ran through the reactor.
Groueff: Yeah. I mean this canal or whatever it was.
Babcock: The water was heated up far short of the boiling point. I don’t even know what it was now, but the water was hot, but not boiling. The temperature of the Columbia River was raised an amount that I expect you could measure it if you had precise instruments. But that’s about it.
Groueff: I see.
Babcock: A hundredth of a degree maybe? I don’t know what it was.
Groueff: I see—nothing that the population down the river would notice?
Babcock: Nothing that the population down the river would know. However, if a person were allowed to swim near the exit where this water discharged into the river, they could find a local area that would be warm, yes. But there was no problem there.
Groueff: I wanted to ask you, I read in the book of Dr. Compton that at certain moments of the operation, the whole chain reaction stopped because of this problem of the xenon. Then he talks about the man who solved this problem, and he says he’s an anonymous engineer of DuPont, and he calls him, quote, “Old Moss George.” Now who was this man?
Babcock: That’s Dr. George Graves, who I mentioned was the assistant director of—
Groueff: Dr. George Graves. I see. So he was responsible for, I mean, he built the plant in a way that—
Babcock: You better read my article there.
Groueff: Oh you have an article of that?
McNeight: There have been some misconceptions on this in previous writings, and Dale has attempted to do some clarification in this.
Groueff: Oh, so you did the whole article on xenon? That’s perfect. That’s exactly what I want. Tell me, from your experience before and after the Manhattan Project, how would you rate the job done during the Manhattan Project? Is it the biggest job ever done?
Babcock: At that time, it was certainly the largest job that the DuPont Company had ever undertaken.
Groueff: The largest?
Babcock: The largest.
McNeight: Certainly as of that date, it ranked amongst–I’ll say the most difficult process operation jobs that probably anyone had ever.
Babcock: If you will exclude other operations that were going on at the same time, such as the Oak Ridge.
McNeight: Well this is why I used the specific words, Dale, as of that time, it was amongst the most difficult.
Babcock: There’s no doubt about that.
Groueff: Some other difficulties I’ve found were mentioned, like the extremely difficult problem of welding of the steel plates surrounding the piles. I don’t know what that is. Also the problem with shielding for the piles was quite hard.
McNeight: I don’t recognize the welding problem.
Babcock: Well, there was a welding problem. Let’s see if I can explain what it was.
McNeight: Let me ask one thing. Is Gil Church on the—
Babcock: I was just going to say we’re doing Gil’s at 9:30.
McNeight: He is the proper man to raise that question to, I believe, yes.
Babcock: I was going to say, we’re running out of time here. I’m talking too much.
Groueff: One man that I’m trying to locate and interview is the military head of Hanford.
McNeight: Colonel [Kenneth D.] Nichols?
Groueff: Colonel [Franklin] Matthias.
McNeight: Oh, Matthias. Yes.
Groueff: Do you know whether he’s alive, where he is?
Babcock: I’m reasonable sure he’s alive, but I haven’t heard from him.
Groueff: Do you have a lot to do with the military there?
Babcock: Very minimal.
McNeight: Very minimal.
Groueff: Very minimal.
Babcock: Very minimal. Yes.
Groueff: And General Groves, did he come there?
McNeight: He came there on inspection visits, shall we say?
Babcock: Yes. Moderately, frequently.
Groueff: But for the day-by-day work, who was the boss of Hanford? Who was the man running your people?
McNeight: Well, during the construction phase, Gil Church.
Groueff: He was the boss of the whole place?
McNeight: After the plant was in operation, Walt Simon was the plant manager.
Babcock: We had two or three plant managers.
McNeight: Yeah, but Walt was plant manager at the start.
Groueff: I think that’s interesting.
Babcock: We’re having lunch with Simon.
McNeight: So Church and Simon—
Groueff: There were other people, and Matthias was just from the military side.
McNeight: He was responsible for the Manhattan districts. The Corps of Engineers’ responsibility is there. Now I believe you have some time with Mr. Greenewalt, don’t you?
Groueff: Yes.
McNeight: He would be the right person, I think, to explore the relationships.
Groueff: I see. But while the Hanford was being constructed, your boss was?
McNeight: Strictly DuPont people.
Groueff: Yeah, Church and—
Babcock: No, no.
Groueff: No?
Babcock: You’ll have to get our organization chart. Excuse me. You’ll have to see what our organization chart is. Gil Church was the representative of the engineering department at Hanford who had local responsibility, reporting back to Wilmington through Slim Read and whoever his bosses are.
Groueff: I see.
Babcock: Now Gil Church had the engineering responsibility for erecting the plant.
Groueff: But not for the reactors?
Babcock: Well, yes, he built the reactors. But the DuPont Atomic Energy Division had the overall responsibility of the whole thing. The DuPont engineering department took their process directions from the Atomic Energy Division.
Groueff: I see.
Babcock: Which said, “Build it this way,” and he then built is using the best engineering judgment they could. He built it that way. But the prime responsibility for the Hanford operation rested in the Atomic Energy Division—the head of which was Mr. Roger Williams.
McNeight: The sound relationship of the architect to a contractor in the house.
Groueff: I see.
Babcock: It leaves out the whole plans.
McNeight: Actually, this is the basic company way of working all the time.