The 1975 Report of the Joint Congressional Committee on Atomic Energy concluded that “nuclear medicine represents one of the most successful applications of the peaceful use of atomic energy.” Nuclear medicine harnesses radioactive products to visualize and treat a variety of diseases. This is in contrast to the related field of radiology, which uses externally applied radiation to image and treat diseases. Today, millions of nuclear medicine procedures are performed in the United States every year, where the legacy of the Manhattan Project lives on in the treatment and visualization of disease.
As nuclear medicine and nuclear power spread from behind the walls of the Manhattan Project sites, health physics followed, growing into its own discipline focused on keeping people safe from the harmful effects of radiation.
Health Physics
In the wake of the atomic bombings in Japan, increased scrutiny was placed on the field of health physics. Organizations such as the International Commission on Radiation Protection and the National Council on Radiation Protection and Measurements frequently reduced the permissible exposure limits, and many people turned their attention to the long-term effects of radiation exposure.
In 1949, the United States, Canada, and Great Britain met in Chalk River, Ontario, for a conference on permissible doses of radiation. The Tripartite report was published after the conference, which collected the information on radiation protection known at the time, different measurements of dosage, and standards for radiation protection.
Today, the ALARA concept helps guide the field of health physics. ALARA, which stands for “as low as reasonably achievable,” began to be codified in the 1950s. The ALARA principle, which officially became law in 1977, acknowledges the fact that some radiation may be beneficial while aiming to limit as much as possible any radiation exposure. The development of ALARA marked a shift in thinking about health physics to a mindset that no amount of radiation can be considered safe. This was in contrast to the earlier concepts of a theoretical “tolerance dose” as used by men such as Arthur Mutscheller, which assumed that at low enough doses radiation was not harmful. However, the fundamental tenets of health physics, including the basic tools such as film badges and dosimeters—which measure radiation exposure in a variety of ways—and practices such as minimizing time, maximizing distance, and using appropriate shielding remain the same since the Manhattan Project.
Nuclear Medicine
One of the goals of the Atomic Energy Commission, which was founded in 1946 to oversee the peacetime development of nuclear technology, was to facilitate research into nuclear medicine. Posts were established at laboratories and universities around the country, funded by the federal government. At the same time, a paper on the successful treatment of thyroid cancer using a radioactive isotope of iodine was published in the Journal of the American Medical Association in 1946. This landmark paper helped bring nuclear medicine out from the secret cities of the Manhattan Project and into the national spotlight.
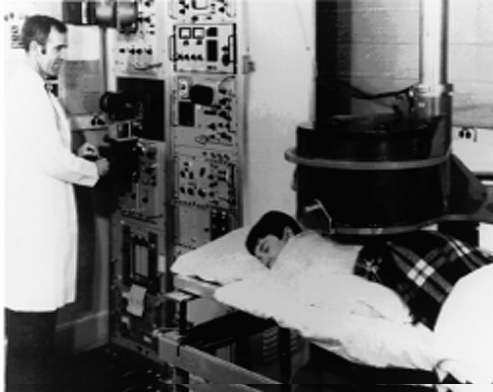
By the early 1950s, nuclear medicine had reached widespread clinical use. This was also due to a program of isotope distribution emanating out of Oak Ridge, Tennessee, that produced a large variety of radioisotopes and supplied material free of charge to cancer researchers. This program, led by Dr. Paul Aebersold, also included substantial educational components to spread isotope production and use into labs around the country.
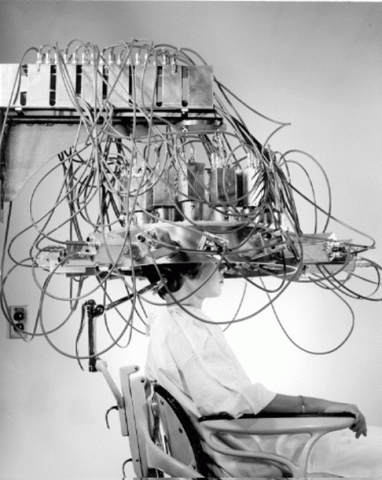
The 1950s also saw a broadening in the scope of nuclear medicine to include medical imaging. The tools used were born from those created in the field of health physics to detect radiation. The rectilinear scanner, which detected radioactive substances in the patient’s body, was invented by Benedict Cassen and Fred Bryan. This scanner was used to meticulously map out the radioactivity emitted from injected radioactive molecules, a process which became automated and standardized to scan the patient and produce a two dimensional picture. These scanners were crucial to the growth of nuclear medicine. Their clinical applications and profitability helped foment commercial interest.
In the 1960s, Hal Anger of the Donner Laboratory at Berkeley invented the scintillation camera, which measured the gamma radiation emitted without the need to scan over each part of the area of interest one by one. By simultaneously imaging the entire area, the Anger camera gradually displaced the earlier scanner. These devices, when paired with injected radioisotopes, allowed researchers unprecedented access to the internal workings of the body. Modern nuclear imaging tools, including PET and SPECT scans, benefit from these scanners.
Nuclear Medicine Today: Imaging
One of the main areas of health physics research during the Manhattan Project was in radiation detection. By building devices to quantify radiation amounts, the medical groups of the Manhattan Project could understand what procedures and protection were required for the men and women who worked on the atomic bomb project. These devices are the forefathers for the most widely used application of nuclear medicine, nuclear imaging, which measures the radiation from specially designed molecules injected into patients to image internal structures.
The two main types of nuclear imaging used today are PET scans and SPECT scans. Unlike many other imaging devices, these tools specialize in depicting how exactly the body functions and not just how it is structured. The SPECT method was invented in the 1950s, but did not come into widespread use until three decades later. PET scans were developed in the 1970s. Today, both types of scans—in conjunction with another scanning technique called computer tomography, or CT scans—are essential tools in diagnosis and the monitoring of treatment.
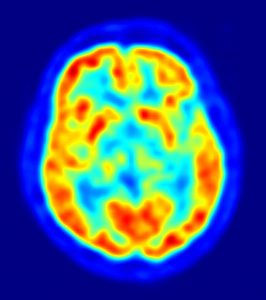
Positron emission tomography, or PET, is a functional imaging tool used to visualize a variety of body functions. A PET scan involves two components, the radioactive tracer and the detector. The radioactive tracer is made up of a molecule normally found in the body fused to a radioactive isotope of fluorine. The selection of the biologically active component depends on what is imaged—it can be a sugar, a protein, or a hormone, for instance. This tracer is introduced into the body, where it interacts with other molecules and structures just as it would without the radioactive component. For instance, PET scans for tumors use a structure that resembles glucose attached to fluorine-18. Glucose is taken up by cells in the body, and fast-growing cells such as cancer cells take up glucose at high rates. The radioactive tracer then becomes localized at high levels in these tumors.
The second component is a 360-degree detector. The radioactive fluorine releases positrons, which have the same properties as electrons but hold an opposite charge. These positrons collide with electrons to produce two high-energy photons that travel in opposite directions. These two light particles strike the detector at two different points, which can then be used to find out where the radiation originated. The PET scan produces a three-dimensional image of the distribution of the tracer molecule in the body, and thus a picture of how the body uses the molecule of interest.
Single-photon emission computed tomography, or SPECT, is an imaging tool that uses gamma rays—high energy light waves—to produce true 3D images of the inside of a patient’s body. SPECT also requires a radioactive molecule and a detector. In SPECT scans, a gamma-emitting radioisotope is used, either alone or attached to another molecule that can guide localization to a structure of interest. Cameras that detect gamma radiation take pictures of the radioisotopes from a variety of angles, building a three-dimensional image that illustrates biological activity. SPECT measures the gamma radiation directly, as opposed to the PET scan’s indirect measurement of radiation. SPECT scans are a cheaper alternative to PET scans, but PET scans provide higher-resolution images.
Nuclear Medicine Today: Treatment
In addition to the diagnostic tools of nuclear medicine, PET and SPECT scans, there are a number of diseases treated by radioactive materials. These treatments are all in addition to those in radiology that use external sources of radiation, including radiation therapies for many cancers.
There are two types of radiotherapy. In unsealed-source radiotherapy, the radioisotopes are introduced into the body, where they localize to specific organs or tissues. Sealed-source radiotherapy keeps the radioisotope contained, either in a capsule or metal wire, at the treatment location. This isolates the radiation to the area of application.
Examples of unsealed-source therapy are numerous. Hyperthyroidism and thyroid cancer are commonly treated using radioactive iodine. Radioactive iodine can also be attached to a biological molecule to treat certain cancers. Radioactive phosphorous is used to treat blood disorders and cases of overactive bone marrow. Yttrium-90 is used to treat a variety of liver cancers. Bone pain that results from cancer is also treated with radioisotopes, including radium-223, strontium-89, and samarium-153.
The most prominent type of sealed-source therapy is brachytherapy, commonly used to treat cervical, prostate, breast, and skin cancer, as well as tumors located in other parts of the body. The radioisotopes commonly used include cesium-131, cobalt-60, iridium-192, palladium-103, ruthenium-106, and radium-226. These radioisotopes are packaged into pellets, which are delivered to the site of interest.
Treatment via internal radiation has a long history, one which stretches back to around the discovery of radiation itself. Nuclear medicine today employs low doses and targeted treatments that benefit from the cross between medicine and radiation. In 1971, the American Medical Association officially recognized nuclear medicine as a medical specialty. Today there are thousands of nuclear medicine centers that perform millions of procedures every year.