In 1913, two American scientists decided to measure the density of water. While not a complicated experiment, the density of water is an important chemical standard that allows for the comparison of densities across different systems and units. The scientists hoped to find a more accurate value, and they assumed it would be an easy task. Their undertaking, it turned out, was not simple. As they measured samples from different locations, they found that the density varied by small amounts. A more accurate measurement was out of reach. The scientists were stumped.
That same year, Frederick Soddy announced his discovery of isotopes—different species of the same element that differ in mass but have the same chemical properties. Though scientists did not realize it at the time, the discrepancies between the weights of the water samples came from different isotopes of hydrogen.
Heavy water, so named for its higher density, consists of water molecules with deuterium isotopes in the place of hydrogen. Deuterium isotopes are composed of one proton and one neutron, while standard hydrogen contains just one proton. Deuterium is therefore twice as heavy as hydrogen, and pure heavy water has a density about 10.6% higher than that of normal water.
Heavy water also has other properties that distinguish it from normal, or “light” water. One of these differences, the lower neutron absorption of heavy water, thrust the material into the center of scientific research during World War II. As scientists decided which materials they would use to build the early nuclear reactors, some staked their country’s nuclear programs on small amounts of a substance practically indistinguishable from water.
History of Heavy Water
By the early 1930s, a number of isotopes of different elements had been detected. However, isotopes were still not well understood; the neutron was still just a concept, yet to be discovered. In 1931, American physical chemist Harold Urey constructed a chart of known isotopes. Gaps in the pattern suggested two additional isotopes of hydrogen and one of helium.

Urey began to look in the atomic spectrum of hydrogen for these isotopes. It was predicted that the isotope with a mass of two (deuterium) would have a small shift in the spectral pattern, and in order to visualize this prediction better Urey attempted to enrich the heavier isotope by distilling liquid hydrogen. On Thanksgiving Day of 1931, his analysis confirmed the discovery of a heavier hydrogen isotope.
The next year, Urey worked on other methods for enriching heavy water, namely though electrolysis. He attempted to make a sample of pure heavy water, but G. N. Lewis, his former mentor at the University of California, Berkeley, became the first to produce pure heavy water in 1933. The next year, Urey won the Nobel Prize in Chemistry for his “discovery of heavy hydrogen.”
Heavy water quickly became an important tool for researchers around the world. Some researchers hoped to utilize its analytical potential in chemistry and biology as a source of neutrons and as a chemical tracer. Others believed it could be used as a treatment for cancer. At Cavendish Laboratory in England, heavy water was used to discover an even heavier isotope of hydrogen called tritium. George de Hevesy used heavy water to calculate the water content of the human body. During World War II, however, heavy water’s most important role would come in the field of reactor physics.
Heavy Water Production
 In the years after its discovery, pure heavy water was a scarce resource. While electrolysis was a simple and effective method of producing heavy water, it required a large amount of energy, and thus special equipment and a large amount of money, to prepare pure samples of significant volume. It soon became clear, however, that the kind of facility required to make heavy water in bulk already existed.
In the years after its discovery, pure heavy water was a scarce resource. While electrolysis was a simple and effective method of producing heavy water, it required a large amount of energy, and thus special equipment and a large amount of money, to prepare pure samples of significant volume. It soon became clear, however, that the kind of facility required to make heavy water in bulk already existed.
In the early twentieth century, scientists searched frantically for a way to fix atmospheric nitrogen into nitrates. The Chilean nitrate deposits, on which the world depended for fertilizer, were running out, and widespread food shortage and famine seemed likely without a new source. In 1902, Kristian Birkeland accidentally discovered a way to produce nitrogen oxides when a device he was working on exploded in the laboratory. Together with Sam Eyde, a civil engineer, Birkeland helped design the Norsk Hydro hydroelectric plant in the Norwegian mountains. While Birkeland’s method was soon replaced with the new Haber-Bosch process, the plant remained a leading producer of ammonia. Before long, ammonia plants were built around the world.
After the discovery of heavy water, Jomar Brun, the head of hydrogen research at Norsk Hydro, and Leif Tronstad, a Norwegian physicist, developed a plan to adapt the plant’s machinery to produce heavy water using electrolysis. By early 1935, the plant was selling 99% heavy water for about 50 cents per gram.
Heavy Water in Reactors before World War II
In 1937, Hans von Halban and Otto Frisch observed a lower rate of neutron absorption in heavy water than that of normal water. The next year, uranium fission was discovered by Otto Hahn and Fritz Strassmann. The two suggested that the process could release neutrons, which had the potential to begin a chain reaction if they could be slowed down. Working with Frédéric Joliot-Curie and Lew Kowarski, von Halban observed these neutrons in April of 1939. A few months later, they found that blocks of uranium oxide displayed increased fission activity when immersed in water. The absorption of the released neutrons by the water, however, prevented the creation of a self-sustaining reaction.
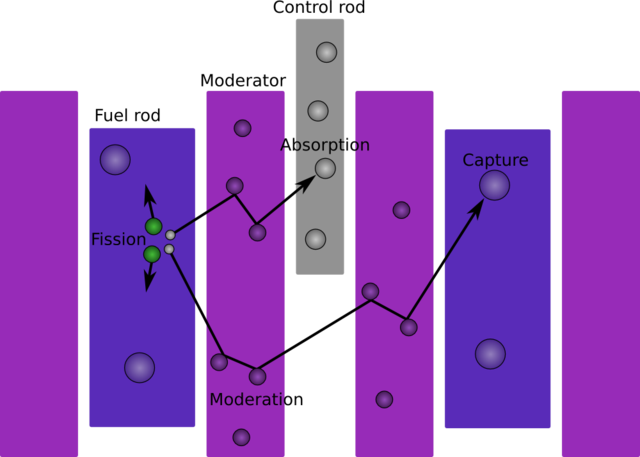
As Hahn and Strassmann theorized, and the French team discovered, nuclear reactors require a moderator, which slows down the neutrons to speeds at which fission can occur. The neutrons released during fission travel at speeds around one-tenth of the speed of light, and slowing them down increases the likelihood that they will strike nearby uranium atoms and therefore propagate the chain reaction. Not any material, however, will do. With natural uranium, regular water absorbed too many neutrons to be an effective moderator.
It is now known that only three practical moderators can be used to construct a reactor using unenriched uranium: heavy water, graphite, and beryllium. Graphite was the most common, but it had to be very pure. French and German scientists dismissed graphite as a practicable moderator due to early measurements of the neutron absorption of impure graphite. Heavy water, a much better moderator, was in short supply. Thus, in Europe a dispute emerged over the major producer of heavy water, the Norsk Hydro plant in Norway.
Heavy Water in Europe
![Rjukanbanen (Rjukan Train Line) from Rjukan towards the power plant at Vemork, Norway. Photo Credit: Anders Beer Wilse [Public domain], via Wikimedia Commons.](https://ahf.nuclearmuseum.org/wp-content/uploads/2017/07/Rjukanbanen_towards_Rjukan.jpeg) In March of 1940, a French intelligence officer named Jacques Allier arrived in Oslo to negotiate with the Norwegian plant. The French military intelligence bureau had learned of considerable German interest in the plant and its heavy water, and hoped to acquire a deal giving them priority. After securing a claim to all future heavy water produced at the site, the French officer packed up all of the plant’s current supply. Afraid that he was being watched by German agents, he booked two flights, one to Scotland and one to Amsterdam. The flight to Amsterdam was forced to land in Hamburg, where it was searched. The officer and his cargo, however, was on the other flight, and from Scotland he made his way through England and back into France.
In March of 1940, a French intelligence officer named Jacques Allier arrived in Oslo to negotiate with the Norwegian plant. The French military intelligence bureau had learned of considerable German interest in the plant and its heavy water, and hoped to acquire a deal giving them priority. After securing a claim to all future heavy water produced at the site, the French officer packed up all of the plant’s current supply. Afraid that he was being watched by German agents, he booked two flights, one to Scotland and one to Amsterdam. The flight to Amsterdam was forced to land in Hamburg, where it was searched. The officer and his cargo, however, was on the other flight, and from Scotland he made his way through England and back into France.
The French scientists continued their research with the new influx of heavy water, but as France faced defeat the scientists were forced to abandon their laboratories in the capital. Protecting the heavy water became their new mission. From the capital, the heavy water was transported to a spa town in central France, where it was hidden in a women’s prison and then in a condemned cell. As France was evacuated, prisoners helped load the heavy water onto a truck, where it was driven to a British coal ship waiting at a French port. The heavy water was loaded aboard, where it was strapped to pallets that would float in case the ship sank. The harbor was bombed, but the heavy water reached London, where it was housed in another prison.
Joliot-Curie stayed in France while von Halban and Kowarski settled at the Cavendish Laboratory to continue their heavy water research. Their experiments indicated that a self-sustaining chain reaction could be achieved with as little as three to six tons of heavy water. While producing heavy water in Britain was briefly considered, the group instead requested the materials from the United States. Heavy water production in North America began, but as the United States entered the war, the center of nuclear research also moved across the Atlantic. The European group moved to Montreal, where they continued periodic collaboration with Americans working on the Manhattan Project. Their research, however, experienced many delays, and they restarted their heavy water research in earnest in 1944.
In Germany, leading experimental nuclear physicist Walther Bothe performed experiments in the summer of 1940 that led him to conclude that graphite would not make an effective moderator without further refining steps, which were ruled out due to cost. This evaluation was not questioned—due in part to the Germans’ deference to authority—and so Germany’s wartime research concentrated on heavy water. The Germans also lacked the industrial effort required for uranium isotope separation, which made highly pure heavy water even more essential.
 In April 1940, Germany invaded Norway. Norsk Hydro fell under German control, and production was ramped up to meet the demands of its scientists. The next year, the Germans forced the Norwegian government to install a catalytic exchange plant at the site to increase heavy water production as their contribution to the Axis war effort.
In April 1940, Germany invaded Norway. Norsk Hydro fell under German control, and production was ramped up to meet the demands of its scientists. The next year, the Germans forced the Norwegian government to install a catalytic exchange plant at the site to increase heavy water production as their contribution to the Axis war effort.
In late 1942 and early 1943, British and American forces launched a series of raids on the Norwegian plant, hoping to cut off the Germans’ supply of heavy water. The Germans were forced to move the heavy water from Norway, and their plans to build a plant in Germany were continually stalled. As air attacks over Germany continued, Werner Heisenberg and his team moved to a small town named Haigerloch near the Swiss border, where they built a lab on the side of a cliff. They were still attempting to reach criticality with their reactor at Haigerloch when the Alsos Mission arrived.
The German nuclear program during World War II had many flaws. There was a significant separation between scientific disciplines, a stark contrast to the collaboration of the Manhattan Project—which, for instance, led Fermi and Szilard to understand that it was the impurities in graphite that made it a poor moderator. In addition, many of Germany’s best scientists had fled the country, and those who remained often focused on rocketry. This was in part due to the German government failing to properly support the project and instead focusing its resources on missile development. Their reliance on heavy water was another nail in the coffin; the Germans eschewed graphite in favor of heavy water, but never controlled enough to get a successful reactor off the ground.
Heavy Water in the United States and Canada: The P-9 Project
 In 1939, Enrico Fermi and Leo Szilard investigated potential moderators in the laboratories of Columbia University. Fermi found that graphite worked marginally well, and Szilard, who had studied chemical engineering before becoming a physicist, suggested that the problem was boron impurities resulting from the boron carbide electrodes used to manufacture graphite. Boron absorbs neutrons at very high rates, and its presence thus “poisoned” the graphite. Fermi and Szilard met with American graphite manufacturers in an attempt to obtain purer stocks of graphite. In December of 1940, the two met with Herbert G. MacPherson and Victor C. Hamister of National Carbon, who helped develop a method to produce graphite practically free of boron for use in Fermi’s reactor studies. On December 2, 1942, Fermi’s Chicago Pile-1 reactor went critical using purified graphite as a moderator.
In 1939, Enrico Fermi and Leo Szilard investigated potential moderators in the laboratories of Columbia University. Fermi found that graphite worked marginally well, and Szilard, who had studied chemical engineering before becoming a physicist, suggested that the problem was boron impurities resulting from the boron carbide electrodes used to manufacture graphite. Boron absorbs neutrons at very high rates, and its presence thus “poisoned” the graphite. Fermi and Szilard met with American graphite manufacturers in an attempt to obtain purer stocks of graphite. In December of 1940, the two met with Herbert G. MacPherson and Victor C. Hamister of National Carbon, who helped develop a method to produce graphite practically free of boron for use in Fermi’s reactor studies. On December 2, 1942, Fermi’s Chicago Pile-1 reactor went critical using purified graphite as a moderator.
While the reactors at Hanford and Oak Ridge were modeled after Fermi’s pile and used graphite as a moderator, the United States continued its research into heavy water-moderated reactors. The Office of Scientific Research and Development put physicist Hugh Taylor in charge of this research, and in October 1942 the effort was codenamed the P-9 Project. The goals of the project were twofold: to provide heavy water for reactors that could be used to produce plutonium if the Hanford reactors failed, and to investigate further the properties of the material. It was known that the Germans were working to produce heavy water, and the Americans wanted to keep abreast of research in case another potential use was discovered.
It was determined that only one facility in or near the United States had the existing facilities to suit the production of heavy water: an ammonia plant located in Trail, British Columbia. Operated by the Consolidated Mining and Smelting Co., the plant produced hydrogen in large quantities through electrolysis, a necessary component of heavy water manufacture. The American government leased land from the company and from the Canadian government and began to adapt the facility into a heavy water factory. Construction began on September 1, 1942, and production began in the summer of 1943.
.jpg)
There are many ways to produce heavy water. At Trail, the hydrogen exchange method was employed. Normal water flowed through tall towers, where it interacted with hydrogen gas and a special catalyst designed by Taylor. This process was repeated, and the slightly enriched water was then passed through electrolytic cells, where light water decomposes into hydrogen and oxygen gas at a higher rate than heavy water. Over time, the solution becomes more concentrated with heavy water.
In addition to the plant in Canada, three facilities were built in the United States. In order to speed up construction and cut costs, three Ordnance Works facilities then still under construction were chosen to house the heavy water production plants: Morgantown Ordnance Works near Morgantown, West Virginia; Wabash River Ordnance Works near Newport, Indiana; and Alabama Ordnance Works near Sylacauga, Alabama. These facilities concentrated heavy water using a distillation method, which harnesses the slightly different boiling points of heavy and light water. The output of the three plants was then shipped to an electrolytic finishing plant at the Morgantown facility, where the last step of processing occurred. The three plants were built over the course of 1943.
Combined, the four facilities had an estimated output of 2.6 tons of heavy water per month. However, the operators of all four plants continually struggled to reach these estimates in a timely manner. Experts investigated each plant and suggested solutions, but only the plant at Trail reached its intended output. However, by the end of 1944 it was determined that the P-9 Project had met its goals. The plants had produced enough heavy water and backup reactors were not needed as the Hanford reactors shipped plutonium to Los Alamos. The three American facilities were shut down in the summer of 1945, while the Canadian plant stayed in operation until 1956. By the end of 1945, 81,470 pounds of heavy water were produced at the four facilities.

The heavy water produced by the P-9 Project was used to build three reactors. Chicago Pile-3—which was constructed at Argonne and went critical on May 15, 1944—was the first reactor built using heavy water and unenriched uranium. In addition, two Canadian reactors used the P-9 product. Work by the French-led team in Montreal, delayed due to tensions with American scientists, started again in 1944. After visits to Chicago and assurance from the Americans that they would help build a pile in Canada, the team built two reactors at the Chalk River Laboratories near Ontario. The first reactor was a small zero energy experimental pile (or ZEEP), which achieved criticality on September 5, 1945. Built under the direction of Kowarski, it became the first nuclear reactor outside of the United States. The second reactor, named NRX for National Research Experimental Reactor, went critical in July 1947. When it was constructed, it became the world’s most powerful nuclear research reactor.
After ZEEP went critical, Kowarski returned to France, where he worked with Joliot-Curie on the nation’s first nuclear reactor. Called the Zoé reactor, it was moderated by heavy water from the Norsk Hydro plant and went critical near the end of 1948.
Heavy Water in the Soviet Union
In 1939, Soviet scientists reviewed the work of Joliot-Curie and Fermi and concluded that heavy water and graphite were the two best options for moderating a potential nuclear reactor. The next year, scientists submitted a plan to the Academy of Sciences of the USSR “on the utilization of the energy from uranium fission in a chain reaction.” While heavy water was scarce, uranium was even scarcer; the Soviet Union had no uranium mines.
In 1940, Konstantin Petrzhak and Georgy Flerov observed the spontaneous fission of uranium. After publishing their discovery, Flerov noticed that the names of all the scientists he knew were working on fission were absent from the literature. Believing this omission to be an indication that the West was intensifying research into fission and its military uses, he wrote a letter to Joseph Stalin urging the establishment of a program on nuclear research. In 1942, Flerov also penned a letter to Igor Kurchatov that contained calculations and plans for an experimental atomic bomb.

Armed with this letter and information from Klaus Fuchs on the American efforts, Kurchatov was well informed about nuclear research. While they initially hoped to use heavy water, Soviet scientists were hampered in their efforts to obtain stocks from the United States. The Soviet team turned its attention to graphite, which was available in a relatively pure form. On Christmas Day in 1946, the Soviet graphite reactor F-1 went critical. Similar in design to the early American reactors, the Russian F-1 pile is still operating to this day, making it the oldest operating nuclear reactor.
A separate team worked on a heavy water reactor. A plant was designed and construction began during the war, but heavy water was not produced in large quantities until 1948. In April 1949, a Soviet heavy water reactor went critical.
The Importance of Heavy Water
Heavy water is still a common moderator in nuclear reactors, most notably in the CANDU reactors and in other pressurized heavy water reactors. Outside of reactor physics, heavy water is used in chemistry to help identify the structures of compounds and in biology for studies of metabolism.
The history of heavy water reactors highlights the immense industrial mobilization required by nuclear programs during World War II. Enriching uranium made building reactors easier, but required large facilities like those at Oak Ridge. For countries who could not enrich uranium, heavy water or pure graphite was required. Both, however, involved their own technological difficulties. The Manhattan Project harnessed the power of industry, science, and government to produce not only the atomic bombs, but a number of reactors and production techniques that rapidly advanced the field of nuclear research.





![A historical sample of "heavy water" produced by Norsk Hydro. By Alchemist-hp (talk) (www.pse-mendelejew.de) (Own work) [FAL], via Wikimedia Commons](https://ahf.nuclearmuseum.org/wp-content/uploads/2017/08/Deuterium_oxide_Norsk_By Alchemist-hp (talk) (www.pse-mendelejew.de) (Own work) [FAL], via Wikimedia Commons.jpg)